University of Szeged
Albert Szent-Györgyi Medical School
Foreign Students' Secretariat
Your Education. Our Mission.
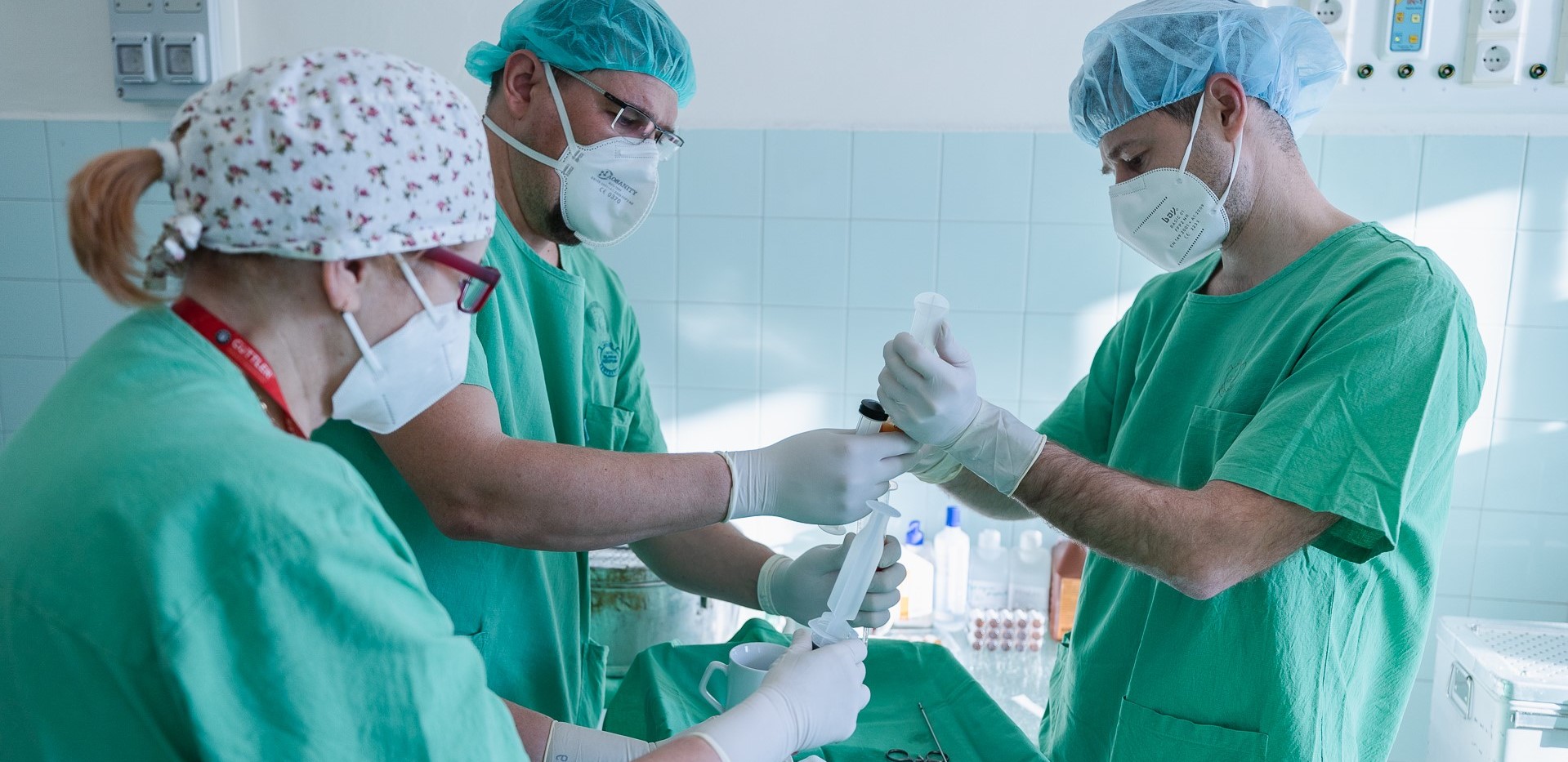
Special Cell Therapy may Provide an Answer to Non-Healing Ulcers at Albert Szent-Györgyi Medical Center, University of Szeged
Difficult or non-healing venous leg ulcers and diabetic leg ulcers can also benefit by a cell therapy procedure that doctors at the Albert Szent-Györgyi Medical Centre of University of Szeged for Department of Dermatology and Allergology are using in a clinical research project. After years of research and development, the therapy will be introduced in Hungary for the very first time through the University of Szeged.
The adipose tissue of the human body contains many cell types with regenerative properties, in addition to fat. Over the last decade, research and clinical trials have been launched worldwide to find out how these cells can be harnessed for new therapeutic applications. Over time, the results have led to the development of a number of therapeutic approaches. Some are already routinely used and others are available through clinical trials. However in Hungary, there are only a few of these procedures currently available. The Albert Szent-Györgyi Medical Centre of University of Szeged for Department of Dermatology and Allergology, as the leading centre in the region, would like to change this by providing care, initially for this region.

The current clinical trial is open to 15 patients from the Department of Dermatology and Allergology, Albert Szent-Györgyi Medical Centre of University of Szeged. Ten of them suffer from venous leg ulcers and five from diabetic leg ulcers.

"In this clinical trial, we use a special technology to extract cells from fat tissue, procured during plastic surgery, which is then injected into patients. In other words, patients get their own cells back, injected around the hard-to-heal ulcer. These cells are expected to trigger healing processes that assist wounds and ulcers to close and exfoliate. This process can take weeks, and the results of the clinical trial will reveal up to six months later if the same procedure is required to be repeated. In the case of the clinical trial, the technology required for the processing of adipose tissue (Lorem Cytori, Celution) was provided by Humancell Technologies Ltd., within the framework of scientific and strategic cooperation. The company is also committed to the introduction and application of cell therapy procedures in Hungary. This technology is already used in many countries, but The University of Szeged would like to introduce it as a therapy for the first time in Hungary." - Said Dr Balázs Bende, principal investigator of the clinical trial.

The idea to launch a clinical trial was conceived seven years ago, and over this period of time, the necessary infrastructure and human resources have been carefully co-ordinated through collaborations and tenders. The research and development test results have led to the establishment of the Personalised Medicine Research Infrastructure at the University of Szeged, covering the entire innovation and healthcare value chain, from disease diagnosis to the development of new therapies and bedside applications. The first result of this development is the ongoing clinical trial.
Prof. Dr. Lajos Kemény, head of the Translational Biomedicine Competence Centre, is the head of research, while Dr. Zoltán Veréb and the staff of the laboratory under his leadership are responsible for the production of stem cells. The clinical trial is led by Dr Balázs Bende. The therapy is carried out by plastic surgeons and dermatology specialists of the SZTE SZAKK, assisted by more than 10 medical staff.
SZTEinfo
Photos by: István Sahin-Tóth